
Development of new materials
Yaegaki Biotechnology Inc. has been conducting research and IP development of new materials in cooperation with various research institutes. We would like to introduce some of these new materials (intellectual property) that we can propose to companies that are interested in implementing them in society as original OEM materials for their final products.
Index
Ⅰ.β-Chitin Nanofiber
1-1 Development of Nanofibers from Chitin
1-2 Physical Properties of β-Chitin Nanofiber
1-3 Functional Studies of β-Chitin Nanofiber
1-4 Awards for β-Chitin Nanofiber
Ⅱ. Palmitoleic acid
2-1 Skin microbiome and new materials to improve it
2-2 Regulation of commensal skin bacteria by sebaceous fatty acid (sapienic acid) in humans
2-3 Selective antimicrobial activity and application development of palmitoleic acid (POA)
2-4 Grant supported Palmitoleic Acid Research and Development
Ⅰ. β-Chitin Nanofiber
1.Development of Nanofibers from Chitin
Nanofibers (nanofibers) are defined as fibrous materials with a diameter of 1 nm to 100 nm and a length 100 times larger than the diameter. Currently, cellulose nanofibers (CNFs), which are made from plant fibers (cellulose) broken down to the nanosize, are the most widely used in industry, and a great variety of CNFs are being researched and developed. On the other hand, it is not well known that nanofibers can be produced not only from plants but also from animals.
In plants, cellulose is a polysaccharide polymer composed of many glucose bonds and has a fiber structure. In animals, chitin is a polysaccharide polymer composed of many glucosamine bonds, a nitrogen-containing sugar (amino sugar), which has a fiber structure and is abundant in invertebrates such as crabs, shrimp and insects. The hard shells of crabs and other invertebrates are made of calcium bound to chitin. Chitin is the second most abundant polysaccharide in the world after cellulose, and is said to amount to several hundred billion tons. Chitin is then broken down to a nanosize similar to cellulose while maintaining its fiber structure, which is called chitin nanofiber (chitin NF).
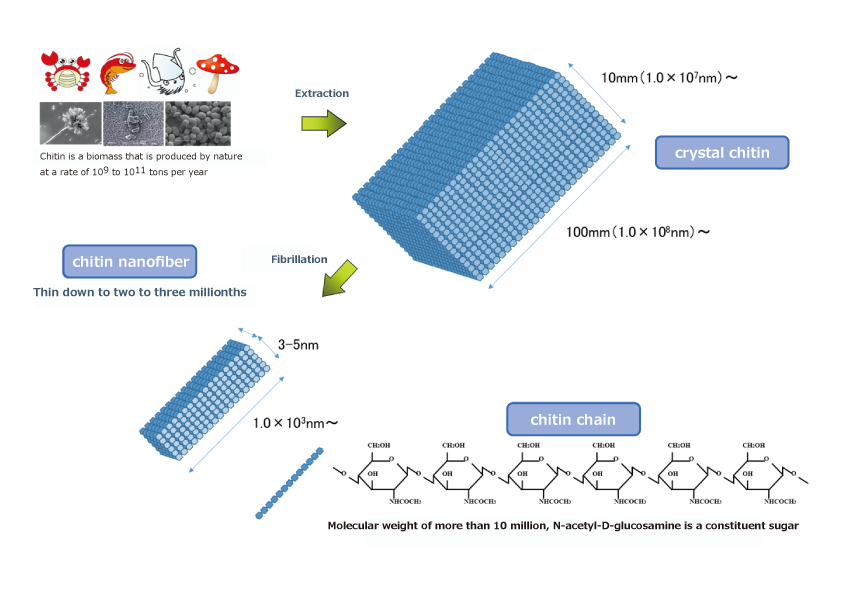
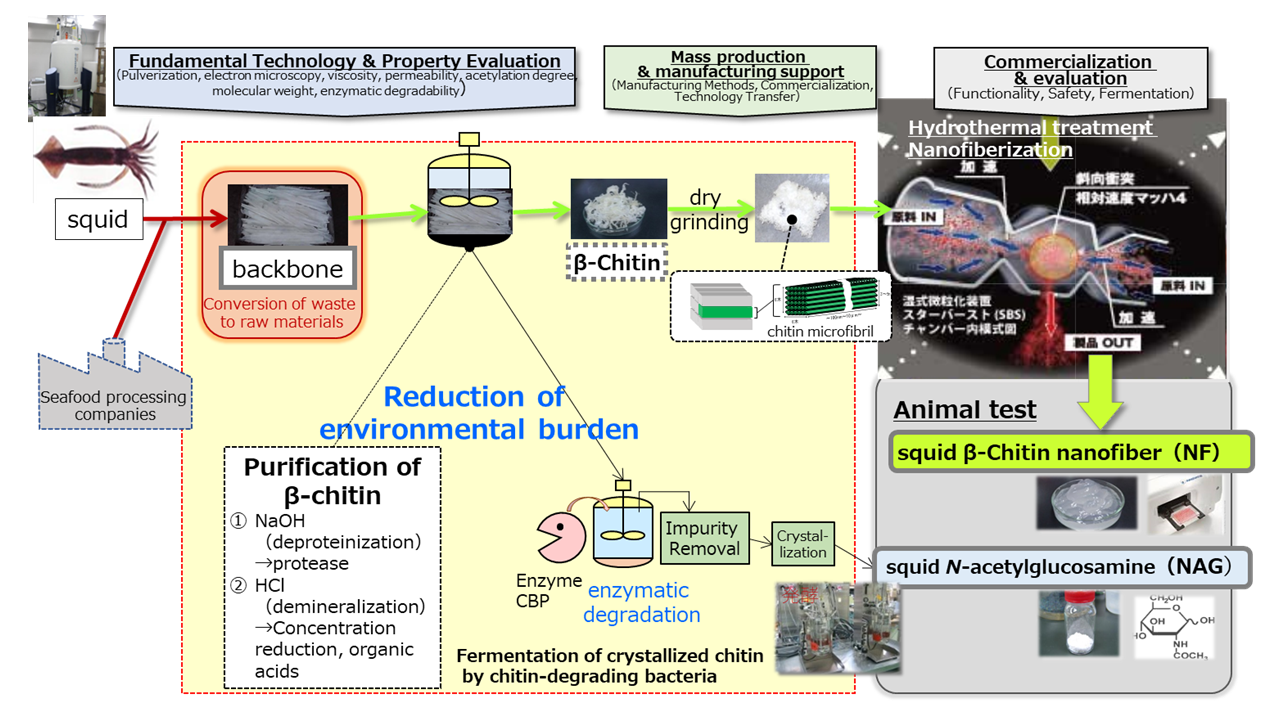
We have focused on squid bones and succeeded in industrial production of β-chitin contained in squid bones as β-chitin nanofibers through the following production process. First, squid bones are subjected to (1) deproteinization (alkaline treatment) in a tank, followed by (2) demineralization (removal of calcium from the bones: concentrated hydrochloric acid treatment) to purify the β-chitin. The resulting β-chitin is then dry-crushed and hydrothermally treated to break down the fibers into fine nanofibers, resulting in β-chitin nanofibers.
After purification of β-chitin, chitin is crystallized by enzymatic degradation and fermented by chitinolytic bacteria as a raw material, which enables us to produce N-acetylglucosamine (NAG), known as a dietary supplement ingredient, with a shorter process, half the production cost and 10 times the production capacity compared to the conventional production method (acid degradation method).

2.Physical Properties of β-Chitin Nanofiber
We found that β-chitin nanofibers (β-chitin NFs) exhibit different physical properties from cellulose nanofibers (cellulose NFs) derived from plants. In particular, β-chitin nanofibers are more biocompatible and easily degraded in vivo compared to cellulose nanofibers derived from plants, making them suitable for medical device auxiliary agents and various biological applications as shown in the table below. Specifically, we have a track record of research and development using β-chitin nanofibers, including the development of microfluidic devices1) and anti-adhesion materials2) in collaboration with universities.
| cellulose NF | β-Chitin NF | |
|---|---|---|
| constituent sugar | D-glucose | N-acetyl-D-glucosamine |
| Fiber Diameter/Length | 10-100nm / 5μm以上 | 3-5nm/1μm以上 |
| Features | Lightweight, high strength, thickening, thixotropic | Lightweight, high strength, thickening, thixotropic, biological properties (AD reduction, IBS improvement, gut health improvement, tissue adhesion, biocompatibility, biodegradability, hemostatic effect, wound healing effect, etc.) |
| biodegradability | non | good |
| allergenicity | non | non |
As shown in the figure below, it was also found that different fiber structures can be obtained by switching the order of (1) protein removal treatment and (2) demineralization treatment in the production process as described in the previous section (see movies below).。
| β-Chitin NF(post-demineralization) | β-Chitin NF(post-deproteinization) | |
|---|---|---|
| appearance | transparence(10 pass) | cloudiness(10 pass) |
| permeability(600nm) | 88%(10 pass) | 68%(10 pass) |
| Viscosity of dispersion | 2000 mPas (10 pass) | 310 mPas (10 pass) |
(References)
1)Ueno et al., Kagaku to Seibutsu 60(1): 44-48 (2022)
2)Nagahama et al., Chitin and chitosan research 28 (2) 90-91 (2022)
3.Functional Studies of β-Chitin Nanofiber
In addition to the uses described in the previous section, β-chitin nanofiber is being investigated for its various functionalities as a food for health maintenance. Here are some of the functionalities.
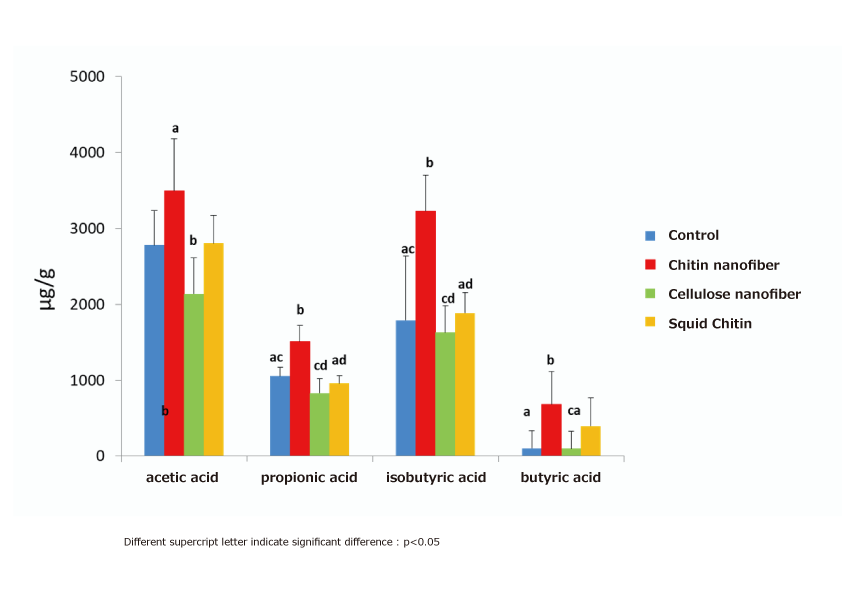
(1) Intestinal regulating action (increase in short-chain fatty acids in the cecum)
Five-week-old male SD rats were divided into four groups and fed a normal diet (control group) and a 1% powdered diet containing β-chitin nanofibers, cellulose nanofibers, and β-chitin for 4 weeks, respectively, and the amount of various organic acids in the cecum contents was measured as changes in the intestinal microflora. The results showed that the β-chitin nanofiber fed group significantly increased propionic acid and isobutyric acid, and tended to increase acetic acid and butyric acid compared to the control group. On the other hand, there was no change in the amount of organic acids in the β-chitin intake group before nanofiberation compared to the control group, indicating that β-chitin nanofibers can change the intestinal microflora and promote the production of organic acids through nanofiberation3).
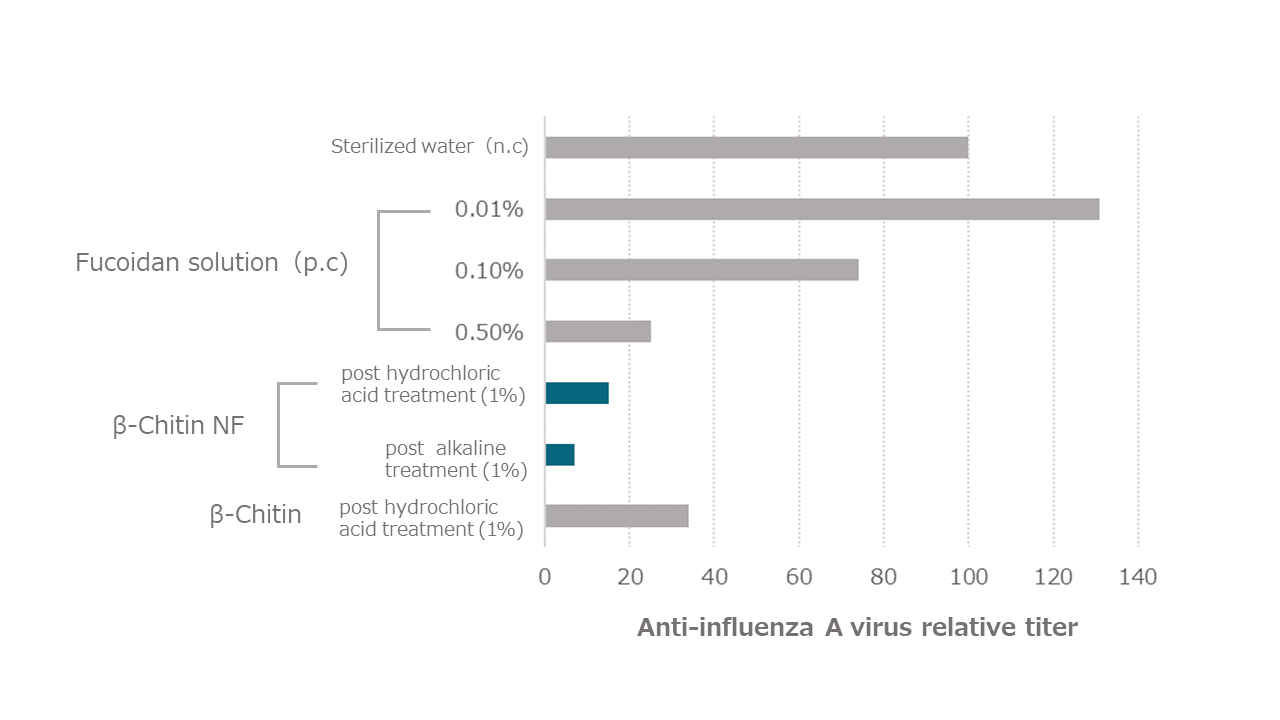
(2) Antiviral effect (influenza virus inactivation)
Canine-derived MDCK cells were seeded, mixed with 250 µL of 1,000 TCID50/mL influenza A virus solution (A/WSN/33 (H1N1)) as the preparation solution and 250 µL of each sample solution, incubated at 25°C for 1 hour, sterile filtered, and added to MDCK cells seeded the day before and incubated at 37°C for 3 days under 5% CO2 (TCID50 assay → Reed-Muench method).
As a result, as shown in the figure below, β-chitin nanofibers (both post hydrochloric acid treatment and post alkaline treatment), which are nanosized from β-chitin, showed strong virus inactivation.
*TCID50:Median tissue culture infectious dose
The concentration at which 50% of the cells are infected by inoculation of the virus diluent on a well plate to which cells have been cultured and adhered.
(Reference)
3)Kazuhiko Yamashita, Chitin and chitosan research 29 (1) 1-4 (2023)
4.Awards for β-Chitin Nanofiber
β-chitin nanofiber has received the 2022 Technology Award from the Japanese Chitin and Chitosan Society as an excellent material from the viewpoint of utilization of unused resources and SDGs. In addition, β-chitin nanofiber and its manufacturing method were selected as “Kansai Monodzukuri Shin-Sen” in 2018, and received the Product and Component Category Award of “Hyogo No.1 Monodzukuri Award” in 2016. If you are interested in OEM production of our β-Chitin Nanofiber, please contact us at the bottom of this page.



Ⅱ. Palmitoleic acid
1.Skin microbiome and new materials to improve it
As it becomes clear that symbiotic bacteria such as intestinal bacteria are deeply involved in the maintenance of health and disease not only in the intestines of the human host but also in the whole body, attention is also being paid to the bacterial microbiome living in epithelial tissues (skin, oral cavity, genital organs, etc.) other than the intestinal tract. Bacteria that are detrimental to human health are called “bad bacteria,” while those that suppress the growth of bad bacteria and promote human health are called “good bacteria”. Good bacteria are utilized in probiotic (live bacteria) products, and in recent years, materials called prebiotics, which work to increase these good bacteria, have been attracting attention. Dietary fibers has been well studied as the representative of these prebiotics. However, Nagao et al. 1) focused on fatty acids, which had not been the focus of attention in the past, as a method to improve the balance of skin bacterial microbiome, and confirmed a natural fatty acid that improves the balance of the indigenous bacterial microbiome of the skin in an extremely outstanding manner.
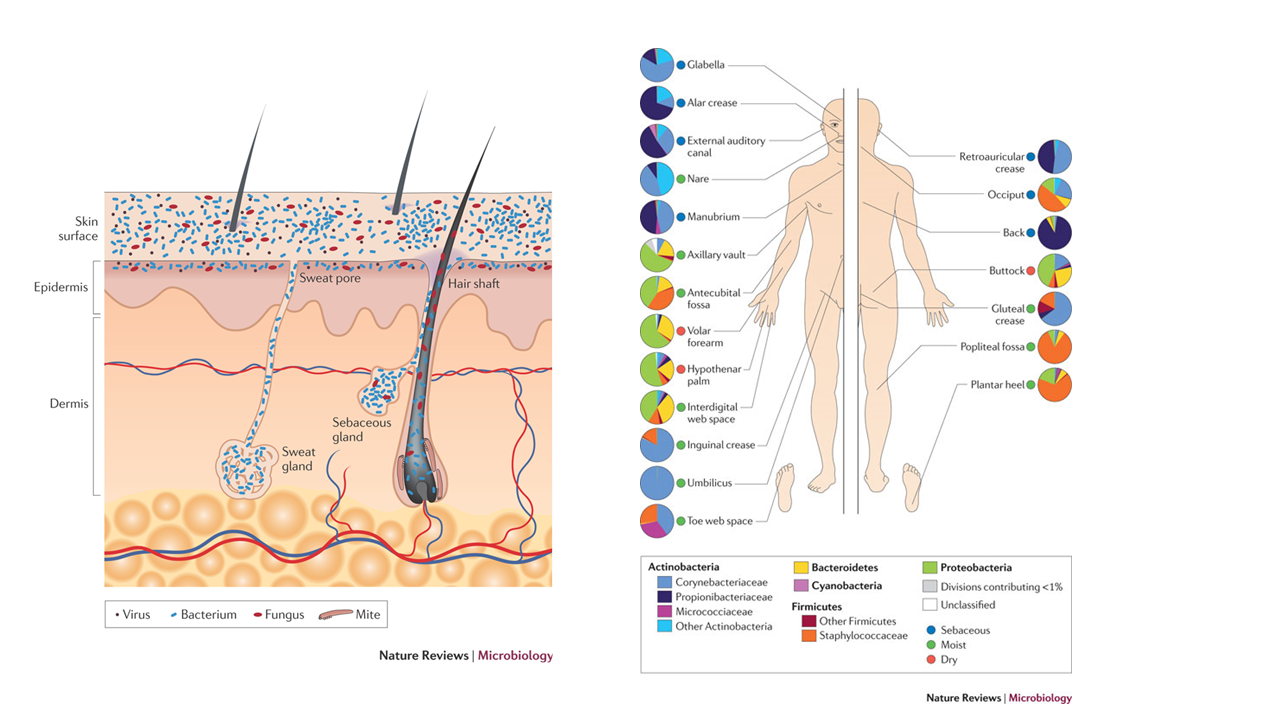
(Reference)
1)Toshihiro Nagao、KAGAKU TO SEIBUTSU Vol. 54, No. 7 (2016)
2.Regulation of commensal skin bacteria by sebaceous fatty acid (sapienic acid) in humans
The composition of the human epidermal flora varies greatly depending on the epidermal area, but the major components are Propionibacterium acnes (now reclassified as Cutibacterium acnes), which secretes propionic acid, a type of short-chain fatty acid, Staphylococcus epidermidis (typical good or opportunistic bacteria), and Staphylococcus aureus (typical bad bacteria). The growth of S. aureus is inhibited in normal human skin by various substances produced by S. epidermidis (e.g., antibacterial peptide AMP, β-defensins, PSM-γ). The lipase secreted by S. epidermidis also breaks down neutral fats secreted by the sebaceous glands into human-specific fatty acids (sapienic acid) and propionic acid produced by C. acnes keep the epidermis in a mildly acidic environment. Sapienic acid is a fatty acid with unique properties not found in other epidermal fatty acids, and is known to inhibit the growth of S. aureus only, which is called selective antibacterial activity. Nagao et al. investigated the selective antimicrobial activity of various types of fatty acids and found that a fatty acid called palmitoleic acid, which is similar to sapienic acid, exhibits similar selective antimicrobial activity to sapienic acid under certain conditions.
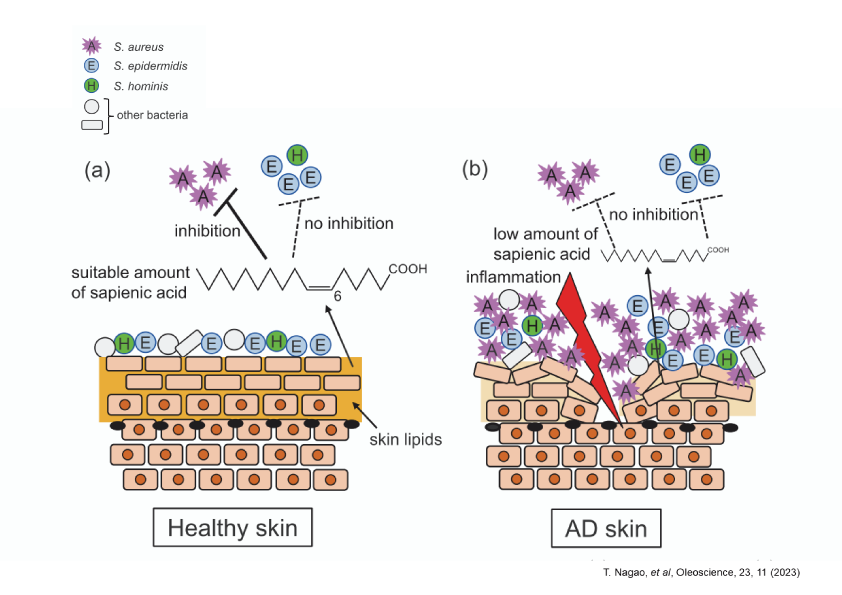
3.Selective antimicrobial activity and application development of palmitoleic acid (POA)
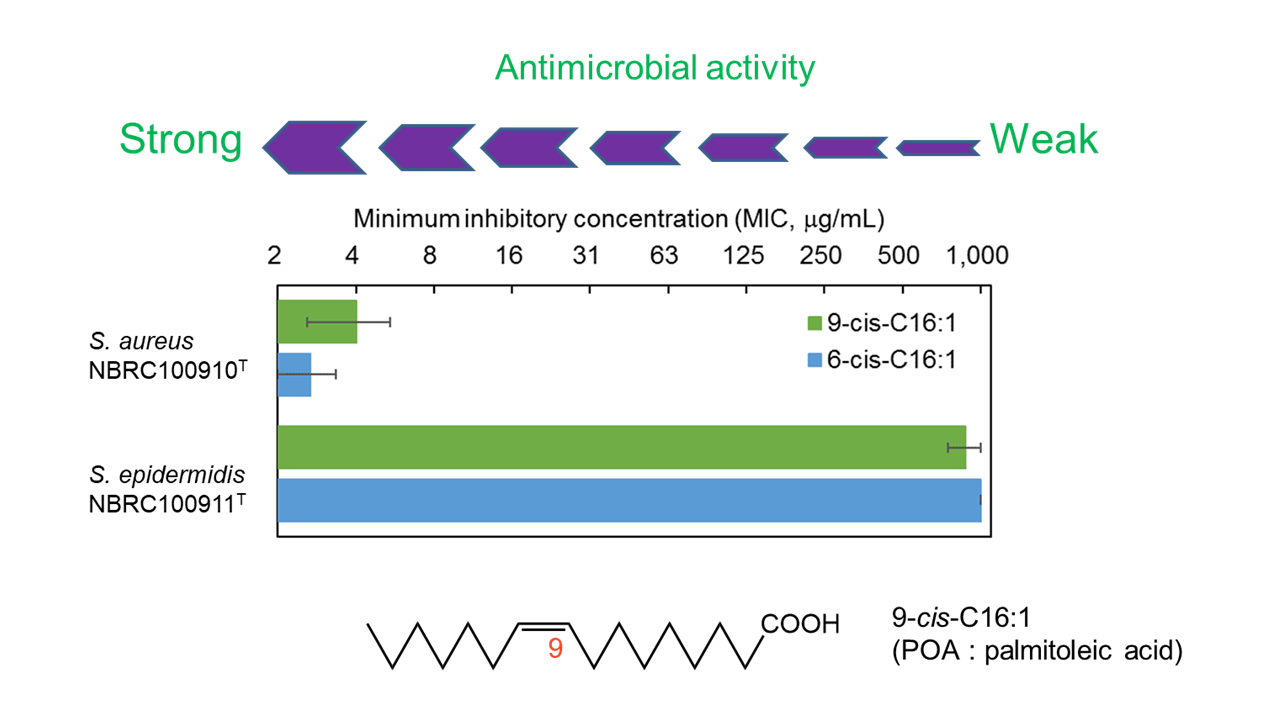
Nagao et al. confirmed that palmitoleic acid (POA) inhibits the growth of S. aureus as well as sapienic acid, but not of S. epidermidis, only in a weakly acidic environment at pH 6.0, as shown in the figure below. Then, What kind of applications can this selective antibacterial activity be used for? One possible application is to patients with atopic dermatitis (AD), where S. aureus tends to proliferate, unlike healthy people, and it has been reported that only strains of S. epidermidis “unable” to inhibit the growth of S. aureus are detected in AD patients, and that the concentration of sapienic acid in the epidermis is less than 1/10 of that in normal people. The cause and exacerbation of AD cannot be explained by this alone, it includes a decrease in epidermal barrier function due to chronic inflammation and activation of the inflammatory cascade (arachidonic acid cascade) due to epidermal dryness, which requires moisturizing, anti-inflammatory measures, and so on. However, it has been suggested that normalization of the bacterial microbiome by application of POA may inhibit the aggravation of inflammation caused by bad bacteria and lead to improvement of skin symptoms in AD patients. As the various functions of indigenous bacteria in health are clarified, it is expected to be applied to a variety of products.
We welcome inquiries about the extraction and purification of POA from plants, and we would be happy to hear from companies interested in OEM production of this technology.
4.Grant supported Palmitoleic Acid Research and Development
The research and development of palmitoleic acid is the result of the following competitive funding, for which we would like to express our deep appreciation.
・Project for Promoting Joint R&D between Public Research Institutes that Play the Gap-bridging Role and Mid-ranking Enterprises and SMEs 2016 (NEDO)
・Strategic Foundational Technology Improvement Support Operation 2020 (METI)
A video interview with one of our researchers is also available for your viewing.